Dr. Rhonda Patrick
@foundmyfitness
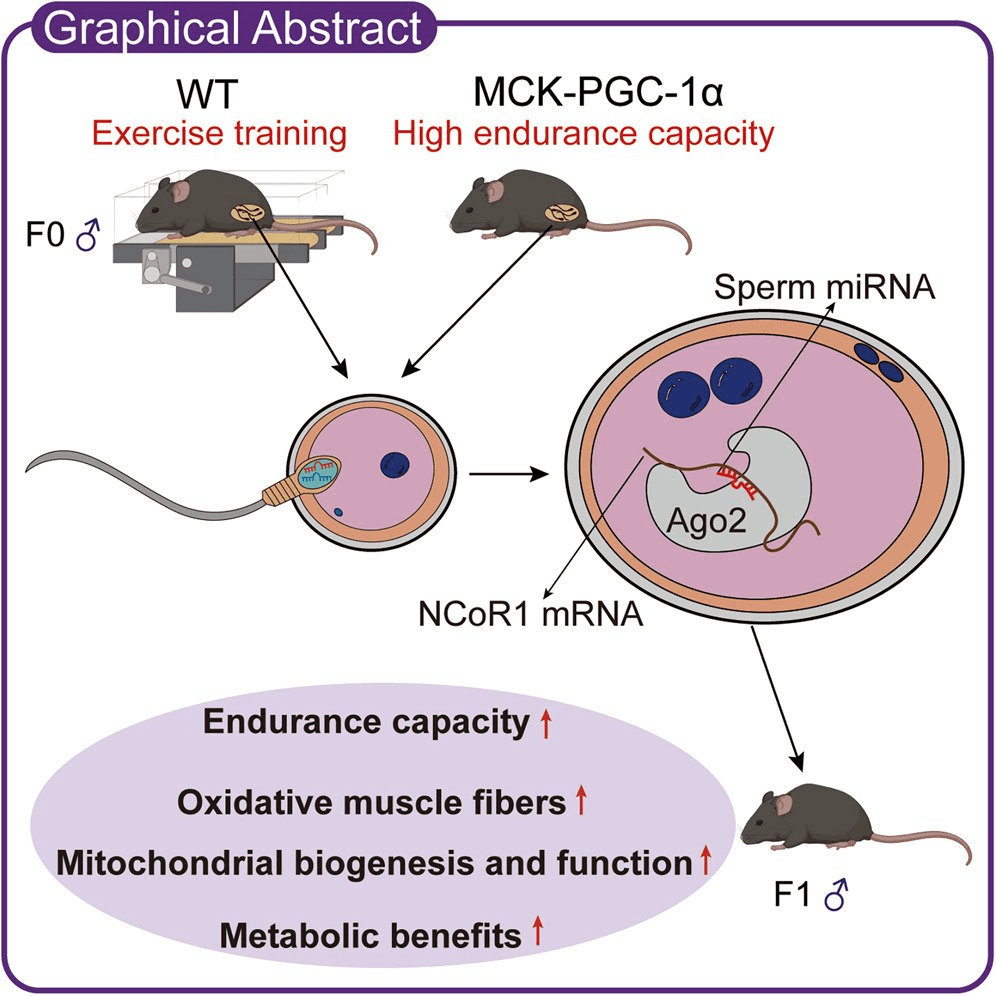
Dad’s workouts may shape his child’s endurance capacity and metabolic health.
Not by passing on mitochondria—which come from mom—but by sending tiny sperm microRNA messages that tune early embryonic gene regulation.
In a new study (mice), exercising fathers produced offspring with a higher VO2 max, greater endurance, a leaner body composition, and more mitochondria. They also had superior glucose control and insulin sensitivity when challenged with a high-fat diet.
Effects tracked to sperm small RNAs that suppressed a “molecular brake” on PGC-1α, the classic mitochondrial biogenesis switch.
This reframes preconception health as a two-parent story: mom supplies the mitochondria, but dad’s training status can still program how those mitochondria are used.
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(25)00388-2
Paternal exercise confers endurance capacity to offspring through sperm microRNAs
Xin Yin1,2,3,4,5,9 ∙ Azhar Anwar1,9 ∙ Linbo Yan1,9 ∙ … ∙ Tao Zhang6 zhangtaocjh@njmu.edu.cn ∙ Chen-Yu Zhang1 cyzhang@nju.edu.cn ∙ Xi Chen1,2,3,10 xichen@nju.edu.cn … Show more
Affiliations & Notes
Article Info
Cover Image – Cell Metabolism, Volume 37, Issue 11
Download PDF
Cite
Share
Set Alert
Get Rights
Reprints
Previous article
Next article
Show Outline
Highlights
•
Paternal exercise enhances endurance capacity and metabolic health in adult offspring
•
Sperm microRNAs regulate embryonic NCoR1 to transmit exercise-induced phenotypes
•
Paternal exercise provides a cost-effective route to improve offspring health
Summary
Paternal exercise influences exercise capacity and metabolic health of offspring, but the underlying mechanisms remain poorly understood. We demonstrate that offspring sired by exercise-trained fathers display intrinsic exercise adaptations and improved metabolic parameters compared with those sired by sedentary fathers. Similarly, offspring born to transgenic mice with muscle-specific overexpression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a booster of mitochondrial function, exhibit improved endurance capacity and metabolic traits, even in the absence of the inherited PGC-1α transgene. Injecting sperm small RNAs from exercised fathers into normal zygotes recapitulates exercise-trained phenotypes in offspring at the behavioral, metabolic, and molecular levels. Mechanistically, exercise training and muscular PGC-1α overexpression remodel sperm microRNAs, which directly suppress nuclear receptor corepressor 1 (NCoR1), a functional antagonist of PGC-1α, in early embryos, thereby reprogramming transcriptional networks to promote mitochondrial biogenesis and oxidative metabolism. Overall, this study underscores a causal role for paternal PGC-1α, sperm microRNAs, and embryonic NCoR1 in transmitting exercise-induced phenotypes and metabolic adaptations to offspring.
Leave a comment